U.S. Department of
Justice
Drug Enforcement Administration
METHYLPHENIDATE
(A Background Paper)
October 1995
Drug and Chemical Evaluation Section
Office of Diversion Control
Summary
Methylphenidate
is a Schedule II stimulant which is structurally and
pharmacologically similar to the amphetamines. It is indicated
for the treatment of Attention Deficit/Hyperactivity Disorders
(ADHD) and narcolepsy. Approximately 85 to 90 percent of all
prescriptions for methylphenidate are written for young children and
adolescents for the treatment of ADHD. Methylphenidate is
available as the brand name product", default", Ritalin, manufactured by
Ciba-Geigy, and as generic products manufactured by MD
Pharmaceuticals.
The use of
methylphenidate in the United States has increased dramatically in
recent years. Since 1990, there has been a six-fold increase
in the U.S. production and utilization of methylphenidate.
This increase contrasts sharply with trends in medical practice seen
in the rest of the world. According to the United Nations 1993
statistics on psychotropic substances (the latest data available
from that body), the U.S. produces and consumes five times more
methylphenidate than the rest of the world combined.
Internationally,
methylphenidate is listed in Schedule II of the Convention on
Psychotropic Substances, 1971, along with amphetamine and
methamphetamine. Under treaty obligations, the United States
must provide the United Nations International Narcotics Control
Board (INCB) with data on the production, distribution and
consumption of methylphenidate. The INCB has, on two recent
occasions, written letters to U.S. officials expressing their
concern about the sharp increase in the use of methylphenidate in
the United States and has requested data on the legal requirements
for the use of methylphenidate as well as data concerning trends in
abuse and possible diversion from licit sources.
While stimulant
pharmacotherapy for the treatment of ADHD in children is recognized
by medical experts worldwide, no other nation prescribes stimulants
in such volume to its children. Epidemiological data indicate
that from 3-5 percent or more of all U.S. children are treated with
methylphenidate for ADHD, frequently without the benefit of other
services as recommended in treatment guidelines.
Support and
advisory groups play an important role in the distribution of
information regarding ADHD and its treatment. In recent years
there have been large increases in membership in these organizations
and participation in their activities. Children and Adults
with Attention Deficit Disorder (CHADD) is the nation’s largest ADHD
support organization. CHADD has a membership of over 28,000
and has 600 chapters nationwide. CHADD sponsors parent support
groups, convenes meetings featuring speakers, works with local
school systems and provides information regarding ADHD related
issues.
It has recently
come to the attention of the DEA, that Ciba-Geigy (the manufacturer
of the methylphenidate product marketed under the brand name
Ritalin) contributed $748,000 to CHADD from 1991 to 1994. The
DEA has concerns that the depth of the financial relationship with
the manufacturer was not well-known by the public, including CHADD
members that have relied upon CHADD for guidance as it pertains to
the diagnosis and treatment of their children.
A recent
communication from the United Nations International Narcotics
Control Board (INCB), expressed concern about non-governmental
organizations and parental associations in the United States that
are actively lobbying for the medical use of methylphenidate for
children with ADHD. The INCB further stated that “financial
transfer from a pharmaceutical company with the purpose to promote
sales of an internationally controlled substance would be identified
as hidden advertisement and in contradiction with the provisions of
the 1971 Convention (Article 10, para 2).” In fact, a
spokesman for Ciba-Geigy stated that “CHADD is essentially a conduit
for providing information to the patient population”. The
relationship between Ciba-Geigy and CHADD raises serious concerns
about CHADD’s motive in proselytizing the use of Ritalin.
In conjunction
with the American Academy of Neurology, CHADD has submitted a
petition to reschedule methylphenidate from Schedule II to Schedule
III under the Controlled Substances Act (CSA). CHADD denies
that the financial contributions received from Ciba-Geigy have any
relationship to their action. The basis for this petition is
that methylphenidate has a lower abuse potential than amphetamines
and that Schedule II controls are unduly burdensome on manufacturers
of methylphenidate, physicians who prescribe it and patients who
receive methylphenidate. In accordance with procedures set
forth in the CSA, the DEA has gathered available data regarding
methylphenidate, conducted an initial review of this information,
and submitted our findings to the Department of Health and Human
Services for their scientific and medical evaluation. The DEA
is awaiting their input for consideration in making a final
determination on the scheduling of methylphenidate.
Of particular
concern is that most of the ADHD literature prepared for public
consumption by CHADD and other groups and available to parents, does
not address the abuse potential or actual abuse of methylphenidate.
Instead, methylphenidate (usually referred to as Ritalin by these
groups) is routinely portrayed as a benign, mild substance that is
not associated with abuse or serious side effects. In reality,
however, there is an abundance of scientific literature which
indicates that methylphenidate shares the same abuse potential as
other Schedule II stimulants. Case reports document that
methylphenidate abuse (like other Schedule II stimulants) can lead
to tolerance and severe psychological dependence.
A review of the literature and examination of current
abuse/trafficking indicators reveals a significant number of cases
where children are abusing methylphenidate.
Whereas the
majority of children experience only minor side effects under
medically supervised controlled conditions, there are a significant
number of case reports documenting more severe abuse. These
reports and scientific studies of abuse potential are routinely
down-played, if referenced at all. As a consequence, parents
of children and adult patients are not being provided with the
opportunity for informed consent or a true risk/benefit
consideration in deciding whether methylphenidate therapy is
appropriate.
Another area of
concern is that children under the age of six are being treated with
methylphenidate contrary to labeling guidelines,
in the absence of controlled studies suggesting that this is
appropriate.
In addition, children are remaining on medication for longer periods
of time, frequently into adolescence and adulthood. Given
recent drug abuse trends which indicate that adolescents are abusing
methylphenidate with serious consequences, the above issues require
close consideration by health authorities.
This paper
provides an overview of the growing availability and utilization of
methylphenidate in the U.S. and outlines concerns regarding
methylphenidate in light of its high potential for abuse. In
preparing this paper, many data sources were reviewed including the
scientific and medical literature, United Nations statistics on
psychotropic substances, Drug Abuse Warning Network (DAWN)
statistics and a number of data sources compiled by the DEA on drug
thefts, manufacture and distribution, and investigative case files.
Information was also supplied by law enforcement personnel, various
state agencies and other interested parties.
Background
Overview of Attention Deficit
Disorder
The Merck Manual
defines Attention Deficit Disorder as developmentally inappropriate
inattention and impulsivity, with or without hyperactivity.
ADHD is implicated in learning disorders and is diagnosed four times
more frequently in boys than girls. Despite the frequent
reference to ADHD as a neurobiological disorder, the cause of ADHD
remains unknown.
The primary signs
of ADHD (with or without hyperactivity) are the display of
inattention and impulsivity. ADHD with hyperactivity is
diagnosed when signs of overactivity are obvious. Inattention
is described as a failure to finish tasks started, easy
distractibility, seeming lack of attention, and difficulty
concentrating on tasks requiring sustained attention.
Impulsivity is described as acting before thinking, difficulty
taking turns, problems organizing work, and constant shifting from
one activity to another. Hyperactivity is described as
difficulty staying seated and sitting still, and running or climbing
excessively.
The American
Psychiatric Association Diagnostic Criteria from DSM-IV lists
symptoms of inattention, hyperactivity and impulsivity to be
utilized in the diagnosis of the disorder. In order for a
diagnosis of ADHD to be made, the symptoms must have persisted for
at least 6 months to a degree that is maladaptive and inconsistent
with the developmental level.
Overview of Methylphenidate
Methylphenidate
is a Schedule II central nervous system (CNS) stimulant and shares
many of the pharmacological effects of amphetamine, methamphetamine
and cocaine. An abundance of literature indicates that
methylphenidate is effective in the symptomatic management of
narcolepsy and ADHD.
The beneficial
effects of amphetamine administration to children with hyperactivity
and behavioral problems was first reported in 1937.
Since that time, central nervous system (CNS) stimulants have been
used in the United States for the management of a triad of symptoms
including hyperactivity, distractibility and impulsivity that has
come to be known as Attention Deficit Hyperactivity Disorder (ADHD).
Methylphenidate hydrochloride is the most commonly used
psychopharmacological agent in children for the treatment of ADHD
with about 85 to 90% of all prescriptions of methylphenidate written
for this indication. The first published pharmacological study
on methylphenidate hydrochloride was by Meier in 1954.
Methylphenidate was introduced into therapeutics that same year and
has since become the focus of hundreds of scientific studies.
Approved for use in
the treatment of Attention Deficit Disorders (previously referred to
as minimal brain dysfunction) and narcolepsy, methylphenidate has
also been used experimentally for the treatment of mild depression,
apathetic or withdrawn senile behavior, and drug-induced lethargy.
Methylphenidate is
a CNS stimulant like amphetamine and methamphetamine, and thus
produces a number of effects including dose related increases in
blood pressure, heart rate, respiration and body temperature,
appetite suppression and increased alertness.
Weight loss and growth retardation are common side effects of
chronic methylphenidate pharmacotherapy in youngsters although drug
holidays on weekends and/or summers can usually compensate for these
deficits.
Serious side effects include facial ticks and muscle twitching.
Other adverse effects of methylphenidate, particularly at higher
than therapeutic doses, include excessive CNS stimulation, euphoria,
nervousness, irritability, and agitation.
Psychotic episodes,
violent behavior, tolerance and severe psychological dependence are
also reported when methylphenidate is abused. While it is
uncertain as to how methylphenidate or other stimulants exert their
effects on the CNS to bring about therapeutic efficacy in ADHD, a
number of neurotransmitter systems are altered by both acute and
chronic methylphenidate administration.
In the U.S., there
are now three registered bulk manufacturers of methylphenidate:
Ciba-Geigy which produces under the brand name of Ritalin, MD
Pharmaceuticals which produces generic methylphenidate and the
recent addition of Johnson Matthey who will be synthesizing
methylphenidate for generic manufacture. Methylphenidate is
available (as Ritalin and in the generic form) in 5, 10 and 20 mg
tablets for oral consumption. Ritalin SR and a generic version
are available as sustained release tablets of 20 mg for oral use.
FDA approved
labeling states that methylphenidate is contraindicated in patients
with marked anxiety, tension and agitation since the drug may
aggravate these symptoms. Methylphenidate is contraindicated
in patients known to be hypersensitive to the drug, patients with
glaucoma and in patients with motor tics or with a family history or
diagnosis of Tourette’s Syndrome. In addition, methylphenidate
should not be used in children under six years of age since safety
and efficacy in this age group have not been established.
Trends in ADHD
Treatment in the U.S.
The use of methylphenidate has
increased dramatically in the U.S. in recent years. The
production and use of methylphenidate has increased almost 6-fold
since 1990. For example, the aggregate production quota for
methylphenidate has increased from 1,361 kg in 1985 to 10,410 kg in
1995 with the primary increases occurring in the last five years.
The United States now consumes
more than 80 percent of the total world supply of methylphenidate or
five times more than the rest of the world combined. While
stimulant pharmacotherapy for the treatment of ADHD in children is
recognized by medical experts worldwide, no other nation prescribes
stimulants for its children in such volume. Epidemiological
data indicate that from 3-5 % or more of all U.S. children are
treated with methylphenidate for ADHD, frequently without the
benefit of other services (e.g. behavioral modification training and
psychotherapy) as recommended in treatment guidelines. Boys
are 4 times more likely to be diagnosed with the disorder.
Increased utilization is also supported by information from state
studies, prescription audit systems and studies of patient visits.
World
Perspective
Internationally, methylphenidate
is viewed as having a very high potential for abuse and is listed in
Schedule II of the Psychotropic Convention. Under treaty
obligations, the United States must provide the United Nations with
data on the production, distribution and consumption of
methylphenidate. Methylphenidate is the only psychoactive
substance listed in Schedule II under international treaty whose
worldwide medical use has increased. According to the 1993
United Nations Report on Psychoactive Substances, the worldwide
medical use of methylphenidate has increased from less than 3 tons
in 1990, to more than 6 tons in 1993. This global trend
largely reflects increased consumption of methylphenidate in the
United States.
The United Nations International
Narcotics Control Board (INCB) has, on two recent occasions, written
letters to U.S. officials expressing their concern about the sharp
increase in the use of methylphenidate in the United States and have
requested data on the legal requirements for the use of
methylphenidate (i.e. prescription in accordance with sound medical
practice – Article 9 of the 1971 Convention) as well as data
concerning trends in abuse and possible diversion from licit
sources.
The following chart depicts world
production of methylphenidate. As can be seen, there have been
vast increases in U.S. production of methylphenidate in recent years:
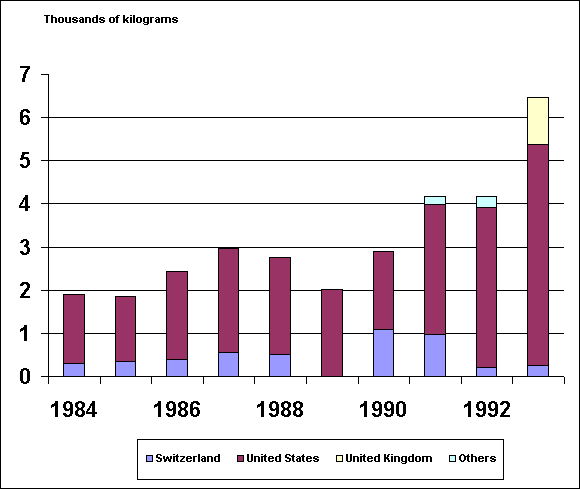
While U.N. data is not yet available,
data for 1994 and 1995 will show substantial increases in U.S.
production of methylphenidate.
The reported worldwide consumption of
methylphenidate is depicted below.
The vast proportion of methylphenidate is consumed by the United
States. In addition, U.S. consumption has increased
dramatically in recent years.
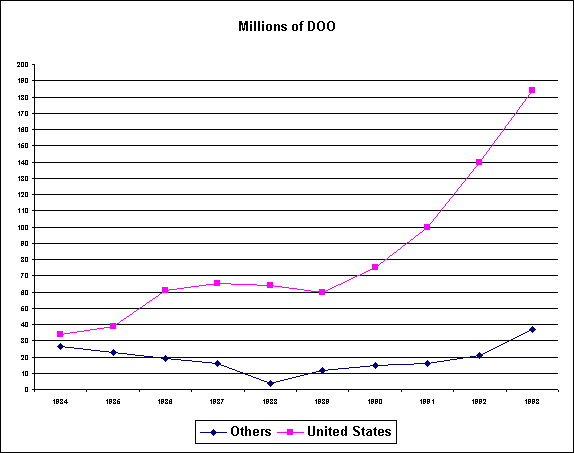
Prescribing
Patterns/Treatment Guidelines
A multimodal approach to the
treatment of ADHD would incorporate the utilization of a stimulant
such as methylphenidate as part of a total treatment program that
includes other remedial measures (psychological, educational and
social) for a stabilizing effect on individuals with ADHD. The
utilization of behavioral therapy in conjunction with drug therapy
is supported, in principle, by most practitioners. While most
practitioners ascribe to such a multimodal approach to the treatment
of ADHD, most children are prescribed methylphenidate chronically as
their sole treatment.
Diagnostic criteria established by
the American Psychiatric Association are not applied uniformly
resulting in some children not being identified as having ADHD and
others being falsely diagnosed with ADHD when other psychiatric
problems may be overlooked. The manner in which a diagnosis of
ADHD is made and the singular treatment approach of psychostimulant
therapy contributes to claims that methylphenidate is overprescribed
and used indiscriminantly in place of disciplinary measures at home
and at school.
Long-term studies indicate that a
multimodal treatment approach is necessary to achieve significantly
improved outcomes for ADHD children. These studies indicate
that treatment with psychostimulants alone does not improve the
outcomes of most ADHD children.
These data suggest that there may be a serious underutilization of
other treatment modalities and that the medical community may not be
meeting the needs of many ADHD children. More promising
outcomes have been reported when multimodal approaches are used in
the treatment of ADHD.
However, data on physician prescribing practices imply that few
general practitioners or pediatricians provide treatment other than
pharmacotherapy with psychostimulants.
Epidemiological data indicate that
U.S. medical practitioners vary greatly in the diagnosis and
treatment of ADHD. One study indicates that a small percentage
of primary care physicians are writing nearly half of all
methylphenidate prescriptions for children.
Another area of concern, is that children under the age of six are
being treated with methylphenidate contrary to labeling guidelines
in the absence of controlled studies suggesting that this is
appropriate.
There is a considerable body of
literature on the short-term efficacy of stimulant pharmacotherapy
on the symptoms of ADHD.
From 60 to 90% of children have been judged as positive drug
responders to methylphenidate medication. However, contrary to
popular belief, stimulants like methylphenidate will affect normal
children and adults in the same manner that they affect ADHD
children.
Behavioral or attentional improvements with methylphenidate
treatment therefore is not diagnostic of ADHD.
Scheduling
History of Methylphenidate
In the United States,
methylphenidate was placed in Schedule II of the Controlled
Substance Act in 1971. This action was based, in part, on a
review by the Department of Health and Human Services (DHHS).
The recommendation by the Secretary reflected advice from the
National Academy of Science/National Research Council Committee on
Problems of Drug Dependence and the Commissioner of the Food and
Drug Administration. Both recommended that methylphenidate be
placed in Schedule II of the CSA. It was found that
methylphenidate’s pharmacological effects are essentially the same
as those of amphetamine and methamphetamine and that it shares the
same abuse potential as these Schedule II stimulants.
While Schedule II regulation
prohibits prescription refills, Federal Law does not limit the
number of dosage units per prescription nor prevent physicians from
issuing several prescriptions at one time as long as they are dated
when the physician issues them.
Quota Setting
Process and 1994 Methylphenidate Shortage
Because methylphenidate is a
Schedule II controlled substance, it is subject to quotas as
outlined in Section 306(a) of the Controlled Substances Act (CSA).
The CSA requires that the Attorney General establish limits or
quotas on the amount of Schedules I and II controlled substances
which may be produced in a calendar year. Quotas take into
consideration the estimated change in medical requirements as
provided by the Department of Health and Human services.
Quotas are established to limit the diversion of drugs from
legitimate channels while ensuring that legitimate medical need is
satisfied. Each year an aggregate production quota (APQ) for
each Schedule I and II substance is set based on sales and inventory
needs. Each company is given a manufacturing quota (MQ) to
provide for these needs. Adjustments may be made at any time
throughout the year provided that adequate material remains within
the APQ. Also, revisions to the APQ are made midyear based on
the previous years’ year-end data. These revisions take into
consideration any changes in the company’s needs up to that point in
the year. Additionally, if these revisions prove insufficient,
an interim notice may be published to satisfy additional legitimate
needs.
The APQ for Schedule I and II
controlled substances is published in the Federal Register as a
proposal for public comment. Subsequently, these quotas are
finalized through a second Federal Register Notice. Since
1983, these Federal Register Notices have required a review by the
Office of Management and Budget (OMB) prior to publication. In
1988 additional reviews before publication of each Federal Register
Notice were required by the Department of Justice, Office of Policy
Development (OPD). Both reviews added to the amount of time
required publish the aggregate production quotas. This was
particularly troublesome in 1992 and 1993 when it took approximately
two months for external reviews before certain quota Federal
Register Notices could be published. Beginning in 1994, these
external reviews by OMB and OPD were eliminated, thereby greatly
reducing the time required for quota revisions.
The Quota
Process and Alleged Shortage
In response to the delay created
by the external review process in revising the 1993 aggregate
production quota (APQ), Ciba-Geigy (the manufacturer of Ritalin)
issued a press release and over 400,000 letters to health care
professionals accusing the DEA of creating an impending shortage of
their product, Ritalin. This was done at a time when it was
known by Ciba-Geigy that a proposal was pending to increase the
methylphenidate quota. The issuance of such statements caused
great concern within the medical community, and created an
environment of panic for parents of children being treated with
methylphenidate. Groups such as CHADD were also notified of
Ciba-Geigy’s allegations. CHADD, in turn, urged parents to
write their Congressional Representatives and to the DEA to voice
complaints regarding DEA creating a shortage. In addition,
many parents rushed to their physicians to get multiple
prescriptions for methylphenidate in order to ensure they had
several months supply on-hand. In short, Ciba-Geigy was
contributing to a situation which promoted the increased sale of
product through panic buying.
It should be noted that in 1993,
DEA set APQs for more than 60 substances and established revised
manufacturing quotas for more than 150 companies. The extended
external review process affected each company yet only one company
making one product chose to accuse DEA of failing to respond to
their needs. All other companies worked with the DEA to ensure
that adequate amounts of their products were available until the
revisions could be completed. As a result of Ciba-Geigy’s
actions, the DEA sampled several distributors and pharmacy chains
which indicated concern over their ability to obtain Ritalin and the
generic form of methylphenidate. DEA could not conclude that a
shortage of Ritalin or the generic form existed. MD
Pharmaceuticals, the other manufacturer of methylphenidate products,
maintained throughout that they had sufficient quota to manufacture
methylphenidate as long as the revision was published and an
increase granted before the end of the year.
Although both manufacturers of
methylphenidate (Ciba-Geigy and MD Pharmaceuticals) were granted
revised quotas late in the year (October), neither company stopped
manufacturing and sales continued. In addition, each company
ended 1993 with inventory on hand.
In 1994 the manufacturing quotas
were initially established and then subsequently revised twice
during the year due to increased demand for methylphenidate.
This is not surprising since there was increased publicity regarding
Attention Deficit Disorder and treatment using Ritalin by CHADD and
other advocacy groups. Both Ciba-Geigy and MD Pharmaceuticals
were granted quotas near the end of 1994 which were the full amount
each company requested. Ciba-Geigy ended 1994 with a
substantial inventory on hand.
Results of GAO
Review
In 1993, an external review
process caused a 2-month delay in publishing the proposed revised
1993 APQs for several controlled substances. This created
concerns about an impending shortage of some forms of
methylphenidate. In response, CIBA-Geigy sent 400,000 letters
to health care professionals and CHADD warned its members and
Congress about this impending shortage. This created a near
panic situation for patients who thought that they couldn’t get
their medicine because they were told that DEA failed to allow
adequate amounts of methylphenidate to be produced.
Fortunately no widespread shortage materialized in spite of the
panic buying which was prompted. As a result of this incident,
however, the oversight and review procedures for the establishment
of quotas have been revised. Additionally a General Accounting
Office (GAO) investigation was conducted in January 1995. The
GAO report indicated that in 1993, all DEA’s quota regulations had
to be reviewed and approved by OPD (a unit within the Justice
Department) and OMB before publication. Because OPD misplaced
the Federal Register for the revision of 1993 APQ’s, including that
for methylphenidate, a 2-month delay in publishing the revised quota
ensued. In February, 1994, OMB declared DEA quota regulations
to be exempt from OMB centralized review. Under this new
procedure, once the DEA Deputy Administrator approves either the
proposed or final quota notices, they are forwarded directly to the
Federal Register for publication. This new procedure
eliminates the cause of the delays in publishing Federal Register
Notifications that occurred in 1993 and there is no reason to
believe that any such delays will occur in the future. Prompt
publication of quota Federal Registers have occurred since the
revised procedures were initiated and no shortages of any controlled
substance have been a result of DEA not providing quotas to meet
medical needs.
Current
Industry Practices\Concerns
CHADD/Ciba-Geigy Relationship
Children and Adults with Attention
Deficit Disorders (CHADD) is the nation’s largest ADHD support
organization. CHADD was begun in 1987 by a small group of
parents and professionals. Today, CHADD has grown to over
28,000 members and 600 chapters nationwide. CHADD works at the
local, state and national levels. On the local level, CHADD
sponsors parent support groups, convenes meetings featuring
speakers, works with local school systems to ensure appropriate
educational services for children with ADHD and publishes local
newsletters. The national office of CHADD provides information
on the latest developments in ADHD related issues.
A DEA review reveals that most of
the ADHD literature prepared for public consumption and available to
parents, does not address the abuse liability or actual abuse of
methylphenidate. Instead, methylphenidate is routinely
portrayed as a benign, mild stimulant that is not associated with
abuse or serious side effects. In reality, however, there is
an abundance of scientific literature which indicates that
methylphenidate shares the same abuse potential as other Schedule II
stimulants. Case reports document that methylphenidate abuse
(like other Schedule II stimulants) can lead to tolerance and severe
psychological dependence.
In addition, a review of the literature reveals cases where children
are abusing methylphenidate.
Whereas the majority of children
experience only minor side effects under medically supervised
controlled conditions, the case reports documenting more severe
abuse and scientific studies of abuse potential are routinely
down-played, if referenced at all. As a consequence, parents
of children and adult patients are not being provided with the
opportunity for informed consent or a true risk/ benefit
consideration in deciding whether to initiate methylphenidate
therapy.
It has recently come to the
attention of the DEA, the Ciba-Geigy (the manufacturer of the
methylphenidate product marketed under the brand name Ritalin)
contributed $748,000 to CHADD from 1991 to 1994.
The DEA has concerns that the depth of the financial relationship
with the manufacturer was not well-known by the public, including
CHADD members that have relied upon CHADD for guidance as it
pertains to the diagnosis and treatment or their children. A
recent communication from the United Nations International Narcotics
Control Board (INCB), expressed concern about non-governmental
organizations and parental associations in the United States that
are actively lobbying for the medical use of methylphenidate for
children with ADHD. The INCB further stated that “financial
transfer from a pharmaceutical company with the purpose to promote
sales of an internationally controlled substance would be identified
as hidden advertisement and in contradiction with the provisions of
the 1971 Convention (Article 10, para 2).”
In 1993 and 1994 when Ciba-Geigy
warned of an impending shortage of Ritalin, CHADD was active in
having its members write their Congressional Representatives to
complain about the situation. In letters to members and
interviews with the media, CHADD officials also were active in
perpetuating concerns that a shortage of Ritalin was imminent.
The DEA received more than 135 inquiries from Congressional
Representatives. In these communications, CHADD routinely
referred to a “Ritalin shortage” as opposed to a “methylphenidate
shortage”. The relationship between Ciba-Geigy and CHADD
raises serious concerns about CHADD’s motive in proselytizing the
use of Ritalin through the use of the brand name as opposed to the
generic name methylphenidate in its literature.
In conjunction with the American
Academy of Neurology, CHADD has submitted a petition to reschedule
methylphenidate from Schedule II to Schedule III under the
Controlled Substances Act. Ciba-Geigy stands to benefit from a
change in scheduling of methylphenidate. However, CHADD denies
that the financial contributions received from Ciba-Geigy have any
relationship to the scheduling petition.
Advocacy Groups and Promotion of
Methylphenidate
Dissemination of Information which is
Inconsistent with Scientific Literature
The documentation in this report
directly refutes the assertions that methylphenidate is a benign,
mild stimulant that is not associated with abuse or serious side
effects. The majority of the literature prepared for public
consumption and available to parents does not address
methylphenidate’s abuse liability or actual abuse. The abuse
reports demonstrate that even adolescents who are abusing
methylphenidate do not view this activity as dangerous.
Whereas the majority of children experience only minor side effects
under medically supervised controlled conditions, as reported
broadly in short-term efficacy studies, the smaller number of case
reports documenting more severe abuse and scientific studies of
abuse potential is down-played , if referenced at all. As a
consequence, parents of children and adult patients are not being
provided with the opportunity for informed consent or a true
risk/benefit consideration in determining if they want their
children or themselves taking methylphenidate.
Current Public Health Concerns:
Abuse
Liability of Methylphenidate
Summary
Methylphenidate is a psychomotor
stimulant structurally and pharmacologically related to the
amphetamines. Studies and case reports indicate that
methylphenidate has the same dependence profile as other Schedule II
stimulants. Like other Schedule II stimulants, abuse of
methylphenidate can lead to tolerance and severe psychological
dependence.
Psychotic episodes, violent behavior and bizarre mannerisms have
been reported.
Intravenous
and intranasal abuse can result in serious medical complications.
Studies
Methylphenidate produces
d-amphetamine and cocaine-like reinforcing effects in both humans
and non-human animals. Preclinical self-administration studies
show that methylphenidate is self-administered by animals
under a variety of conditions, including when substituted for
cocaine or d-amphetamine in drug-experienced animals or when
initiated in drug-naïve animals. Methylphenidate has
reinforcing efficacy similar to cocaine and d-amphetamine. In
non-human primates, methylphenidate can maintain high rates of
self-injection in progressive ratio studies and is chosen over
cocaine in preference studies. In clinical studies
methylphenidate is self-administered by humans and produces patterns
of reinforcing and subjective effects similar to d-amphetamine.
Methylphenidate and d-amphetamine produces similar patterns of
subjective effects, including increases in rating of euphoria, drug
liking and activity and decreases in sedation.
Drug discrimination procedures
provide an indirect measure of a drug’s reinforcing effects and its
abuse potential.
Years of drug discrimination research show that methylphenidate is
(1) discriminable, (2) can be used as a discriminative stimulus
training drug, and (3) generalizes to a number of psychomotor
stimulants including cocaine, and d-amphetamine.
In preclinical studies, chronic administration of methylphenidate
produces tolerance to its disruptive and stimulus effects and shows
cross-tolerance with d-amphetamine and cocaine.
In animals, chronic or acute
administration of high doses of psychomotor stimulants, such as
methylphenidate, cocaine, and d-amphetamine and some substituted
phenethylamines, produce a syndrome of behavioral effects
characterized by aggression, agitation, disruption in food intake,
visual tracking, stereotypies and death.
In humans, methylphenidate
produces behavioral, physiological, subjective, and reinforcing
effects similar to those of d-amphetamine
including increases in rating of euphoria, drug liking and activity,
and decreases in sedation. Methylphenidate produces
stimulant-like discriminative stimulus effects in humans.
Abstinence from stimulants, such
as d-amphetamine and cocaine, after chronic use results in the
appearance of withdrawal signs within one to three days, including
depression, sleep disturbances, anxiety, fatigue, anger/hostility,
dysphoria, psychomotor agitation, confusion and drug craving.
Case studies document the same type of syndrome with methylphenidate
abstinence after chronic use.
Methylphenidate has been used experimentally to alleviate the
abstinence syndrome associated with cocaine dependence.
It is clear that methylphenidate
substitutes for cocaine and d-amphetamine in a number of behavioral
paradigms and there is cross-stimulant sensitivity in animal
studies. Taken together, studies suggest that a similar form
of sensitization may be occurring in humans that are exposed to
stimulants (e.g., methylphenidate) and that this drug history may
predispose individuals to cocaine’s reinforcing effects.
In a study of the incidence of cocaine use and abuse in adult
subjects exposed to methylphenidate as children, medicated ADHD
subjects who tried cocaine reported higher levels of drug dependence
than non-medicated ADHD subjects and controls.
Actual Abuse and Diversion of Methylphenidate
Actual Abuse
A review of the available
literature shows that methylphenidate is associated with patterns of
abuse similar to other Schedule II stimulants. Like
amphetamine and cocaine, abuse of methylphenidate can lead to marked
tolerance and psychic dependence. The pattern of abuse is
characterized by escalation of dose, frequent episodes of binge use
followed by severe depression, and an overpowering desire to
continue the use of this drug despite medical and social
consequences. The abuser may alter the mode of administration
from oral use to snorting or intravenous injection to intensify the
effects of the drug. Typical of other CNS stimulants, high
doses of methylphenidate often produce agitation, tremors, euphoria,
tachycardia, palpitations and hypertension. Psychotic
episodes, paranoid delusions, hallucinations and bizarre behavior
characteristic of amphetamine-like psychomotor stimulant toxicity
have all been associated with methylphenidate abuse. Severe
medical consequences, including death, have been reported.
Case reports document that methylphenidate abuse can lead to marked
tolerance and psychic dependence in children
and adults.
Although the majority of cases cited in the literature pertain to
adult substance abusers, there are indications of adolescent abuse.
The literature indicates that the addiction produced by
methylphenidate abuse is neither benign nor rare in occurrence, and
methylphenidate is more accurately described as producing severe
dependence.
In the petition to reschedule
methylphenidate, petitioners argue that children do not become
dependent on methylphenidate. While that assessment is
essentially true for a vast majority of youngsters that are being
administered therapeutic doses of methylphenidate or d-amphetamine
under a doctor’s order, DEA’s review indicates that children are
abusing methylphenidate and abuse can lead to dependence and
addiction.
Severe medical consequences
including death have been associated with high doses of
methylphenidate and where methylphenidate has been abused by
snorting or intravenous injection.
Like other psychomotor stimulants, utilization of methylphenidate
within normal therapeutic dose ranges for the treatment of
narcolepsy and ADHD are associated with some risks.
Recent data
suggest that pre-exposure to stimulants, including methylphenidate,
in childhood may predispose these same individuals to the
reinforcing effects of cocaine.
ADHD adults have a high incidence of substance abuse disorders.
With three to five percent or more of today’s youth being
administered methylphenidate on a chronic basis, these issues are of
concern.
A significant body of literature
is available that describes the actual abuse of methylphenidate and
consequences associated with that abuse. Some of the earliest
reported abuse cases came out of Sweden
where the widespread abuse of methylphenidate led to its withdrawal
from the Swedish market in 1968.
Early reports of methylphenidate
abuse in the United States are documented in the scientific and
medical literature. Most of the U.S. abuse literature cite
case reports of individuals while limited studies were conducted on
certain groups or populations. Methylphenidate has been abused
orally, intranasally and intravenously. It has been used alone
and in combination with narcotics producing the same kinds of
effects as those seen with amphetamine alone or in combination with
these same drugs. Throughout the 1970’s and 1980’s several
articles in the medical literature documented the serious medical
consequences associated with intravenous abuse of methylphenidate.
A number of papers documented the abuse of Talwin NX and Ritalin
combination that was so prevalent in Kansas City, Missouri and other
cities in the U.S. and Canada.
The prevalence of the use of methylphenidate among heroin addicts
has been reported
as well as the use of methylphenidate among methadone clients.
Two citations in the literature documented the abuse of prescribed
medication in adolescents treated for ADHD.
High School surveys (1994 Texas
School Survey and Monitoring for the Future) indicate an increased
use of stimulants among high school students. Nationally,
about 10% of 1994 high school seniors reported using amphetamines
(designated as Benzedrine, Dexedrine, Methedrine, Ritalin, Preludin,
Dexamyl and methamphetamine, specifically excluding non-prescription
and over-the counter drugs) without a doctor’s order. Of those
reporting using amphetamines nonmedically, 16.6% reported using
Ritalin, up from 7.8% in 1993 and 3.5% in 1992, representing the
greatest increase in use among drugs mentioned. For
perspective, the report of Ritalin abuse by high school seniors
indicates that more seniors in 1994 were using this drug
nonmedically than those prescribed methylphenidate for ADHD.
Additionally, of those seniors that admitted to using amphetamines
without a doctor’s order, 55.9% reported getting a little high to
moderately high while 16% reported staying high for more than seven
hours, indicating a more serious pattern of abuse.
The Drug Abuse Warning Network
(DAWN) indicates that from 1990 through 1993, most DAWN emergency
room mentions for methylphenidate involved whites (75% to 89%) who
were taking the drug orally (90% to 96%) to commit suicide (47% to
67%). A significant number of these estimated episodes, 28 to
40%, were associated with abuse for dependence or psychological
effects. The percentage of episodes involving youngsters
between the ages of 10 and 19 increased from about 24% in 1990 to
about 55% in 1993. Seattle, Washington, Washington D.C., and
Detroit, Michigan reported the greatest percentage of mentions per
100,000 population. About 90% of the mentions in 1990 were for
drug combinations compared to about 60% of the 1993 mentions
suggesting increasing abuse of methylphenidate as a primary drug of
abuse. Among those drugs listed in combination with
methylphenidate, alcohol and at least one narcotic were consistently
ranked among the top five most frequently mentioned. The high
percentage of attempted suicides is consistent with the high
frequency of depression associated with stimulant abuse. As a
point of reference, only 6 DAWN emergency room mentions were
associated with all Schedule III stimulants in 1992, and only one
mention in 1993.
Diversion
Methylphenidate has been in
Schedule II of the CSA since 1971. This schedule provides the
highest level of control available in the U.S. and is intended to
limit diversion and abuse. Despite the unprecedented
availability of other highly abusable stimulants like cocaine and
methamphetamine, methylphenidate is still highly sought after by the
drug abusing population. The abuse data documented herein all
suggest that methylphenidate is abused by diverse segments of our
population (from street addicts to children) and that significant
amounts of methylphenidate have been diverted to illicit use.
Law enforcement data including
STRIDE, theft reports, DEA case reports and reports submitted from
various states indicate that even under Schedule II control,
diversion and abuse of methylphenidate remains a problem in some
segments of our population. Methylphenidate has been targeted
by organized drug traffickers in several states, is among the top 10
controlled drugs involved in drug thefts and is diverted and abused
by health professionals as well as street addicts. At least
two states, Nebraska and Ohio, have experienced significant
diversion and abuse of methylphenidate. The most recent trend
in methylphenidate diversion centers around the use of this drug for
the treatment of ADHD. Cases document parents abusing their
child’s medication, children selling or giving their medication to
classmates and friends, adolescents crushing the methylphenidate
tablets and snorting the powder (two deaths were associated with
this activity in March of this year) and thefts of school supplies
of methylphenidate.
Unlike cocaine, amphetamine and
methamphetamine where illicit manufacture and illegal importation
into the U.S. account for practically all of the available drugs for
abuse, pharmaceutical products diverted from legitimate channels are
the only sources of methylphenidate available for abuse. The
DEA is not aware of any clandestine production of methylphenidate,
which probably reflects its rather arduous chemical synthesis.
Diversion of methylphenidate has been identified by drug thefts,
illegal sales by health care professionals and prescription forgery.
Law enforcement encounters involving illegal activities with
methylphenidate are also good indicators of the scope of its
diversion and trafficking. The control of methylphenidate in
Schedule II, which has the most stringent regulatory requirements
and penalties associated with illegal activity, has certainly
limited diversion and abuse of this drug. Nevertheless, the
following information shows that methylphenidate is diverted and
trafficked in a manner and amount similar to other legitimately
produced Schedule II substances (e.g. morphine, meperidine,
pentobarbital).
DEA maintains a
data base of reports of stolen/missing controlled substances from
pharmacies, practitioners, manufacturers, hospitals/clinics,
distributors and any other licensed handler of controlled
substances.
The following
table shows the total number of reports and mentions (units of
medication, i.e. a bottle of 100, 20mg tablets and a bottle of 500,
10mg tablets would be considered two mentions) for methylphenidate
and other CII substances provided for comparison of activity (data
for 1990 through May, 1995).
|
SUBSTANCE,
CONTROL STATUS
|
NUMBER OF
REPORTS
|
NUMBER OF
MENTIONS
|
|
AMPHETAMINE, CII
|
710
|
1325
|
|
FENTANYL
|
640
|
858
|
|
PHENMETRAZINE, CII
|
34
|
39
|
|
METHYLPHENIDATE, CII
|
1937
|
4592
|
|
MORPHINE, CII
|
2118
|
4163
|
|
OXYCODONE, CII
|
3132
|
6886
|
|
HYDROMORPHONE,CII
|
1247
|
2151
|
|
HYDROCODONE, CII
|
2109
|
4575
|
|
MEPERIDINE, CII
|
2911
|
5380
|
In summary, a total of 1,937
instances of drug theft have been reported for methylphenidate since
1990, most reports were generated from pharmacies and most thefts
were associated with night break ins. An analysis of the data
entered into the system reveals that methylphenidate ranks in the
top 10 most frequently reported pharmaceutical drugs diverted from
licensed handlers.
Where methylphenidate diversion
was documented, activities involved illegal sales of methylphenidate
by health professionals, prescription forgery, and overprescribing
of methylphenidate by physicians and pharmaceutical theft.
Additionally it is important to note that despite Schedule II
controls on methylphenidate and its predominant use in treating
children and adolescents, methylphenidate is associated with the
following types of criminal drug trafficking activities:
1.
Street sales as determined by undercover buys
2.
Multi-state distribution rings
3.
Multi-drug distribution rings (with cocaine, LSD, marijuana,
hydromorphone and diazepam)
4.
Smuggling from Mexico
5.
Distribution to and use by narcotic addicts
While DEA investigators and
laboratory analyses generally involve wholesale level dealers,
state/local investigations provide more information at the retail or
user levels. DEA does not routinely receive summaries of
submissions of drug evidence to laboratories or law enforcement case
reports from state and local agencies. However, a number of
states have provided data to DEA concerning illicit activities with
methylphenidate. Although this information is not from a
systematic survey, it provides further support that methylphenidate
is sought after by segments of the drug abusing community.
In summary, methylphenidate has
been diverted in a number of ways by individuals and organized
groups. Large quantities of methylphenidate have been obtained
illegally by “doctor shoppers”, organized theft rings, ADHD and
narcolepsy scams, forged or altered prescriptions and through
cooperating physicians or pharmacists. At least two states,
Ohio and Nebraska, have identified themselves as having significant
problems associated with methylphenidate diversion. Recent
trends indicate that adolescents are giving and selling their
prescription medication and youngsters are crushing the tablets and
snorting the powder like cocaine. Two deaths in March, 1995
are known to have been associated with this practice.
As noted above, severe medical
consequences have been associated with the abuse of methylphenidate.
The recent trend in the abuse of methylphenidate among adolescents
is particularly alarming because this is the group that has the
greatest access to methylphenidate from legitimate prescriptions.
Adverse
Effects (Short and Long Term)
The potential adverse effects of
methylphenidate and d-amphetamine are almost identical and are
summarized in the table below:
|
Organic
system affected
|
Methylphenidate
|
Dextroamphetamine
|
|
Cardiovascular
|
Palpitation
Tachycardia
Increased blood pressure
|
Palpitations
Tachycardia
Increased blood pressure
|
|
Central nervous system
|
Excessive CNS stimulation
Psychosis
Dizziness
Headache
Insomnia
Nervousness
Irritability
Attacks of Gilles de la
Tourette or other tic syndromes
|
Excessive CNS stimulation
Psychosis
Dizziness
Headache
Insomnia
Nervousness
Irritability
Attacks of Gilles de la
Tourette or other tic syndromes
|
|
Gastrointestinal
|
Anorexia
Nausea
Vomiting
Stomach pain
Dry mouth
|
Anorexia
Nausea
Vomiting
Stomach pain
Dry mouth
|
|
Endocrine/metabolic
|
Weight loss
Growth suppression
|
Weight loss
Growth Suppression
|
|
Other
|
Leukopenia
Hypersensitivity reaction
Anemia
Blurred vision
|
Skin rash or hives
Blurred vision
|
Ahmann et al. (1993) evaluated
Ritalin’s side effects in a randomized double-blind
placebo-controlled cross-over study with 234 children ages 5 to 15
who met the diagnostic criteria for ADHD. Five of the side
effects studied, insomnia, decreased appetite, stomachache, headache
and dizziness, increased during Ritalin therapy even at relatively
low doses (0.3 mg/kg). This data is consistent with other
studies.
Adverse effects of irritability and sadness have not been well
studied, but have been reported in up to 22% of children receiving
stimulant medication.
The effects of methylphenidate on
growth and the induction of motor tics have been matters of concern
and controversy. Safer et al. (1972) was the first to report
growth suppression in children receiving methylphenidate or
dextroamphetamine. Subsequent studies have reported growth
rebound when stimulant medication is temporarily discontinued.
However, the longer the drug treatment, the more severe growth
suppression will be in adolescence and some drug-treated children
are at risk for considerable growth decrements.
Several reports have indicated that tics may be induced or
exacerbated by psychostimulants.
Stevenson and Wolraich (1989) estimated the risk of tic development
in stimulant treated children to be about 1.3% or higher in children
with a family history of Gilles de la Tourette’s disease or other
tic syndromes. Lipkin et al. (1994) reported that
approximately 9% of children with ADHD treated with stimulant
medication develop tics and dyskinesias, with less than 1%
developing chronic tics or Tourette’s syndrome.
The cardiovascular safety of
stimulant therapy in children has been a concern of many physicians
and researchers. Varying alterations in blood pressure and
heart rate after methylphenidate administration have been reported.
A review by Safer (1992) indicated that in 15 controlled studies
using test doses of methylphenidate, a significant elevation of
resting heart rate was found in previously unmedicated children
(mean + 11 beats/min), but with continued drug treatment, only a
minor increase in heart rate was observed (mean + 4 beats/min).
Both systolic and diastolic blood pressure increases have been noted
but are usually minor after oral administration of therapeutic
doses. Large increases in heart rate, diastolic and systolic
blood pressure have been reported following i.v. administration and
cardiovascular toxicity and death have been reported infrequently.
Wang et al. (1994) reported that 0.5 mg/kg i.v. methylphenidate
produced significant decreases in cerebral blood flow (CBF) in 5
healthy male subjects. Decrements in CBF were 25 + 11%
after 5-10 minutes and 20 + 10% after 30 minutes. The
authors concluded that the lack of regional effects suggest that the
decrease in CBF is probably a direct vasoactive property of
methylphenidate and proposed caution in administration of
methylphenidate chronically or to subjects who may already be
cardiovascularly compromised.
The possibility of drug abuse as a
consequence of methylphenidate treatment remains unresolved.
In a review of the literature, Hechtman (1985) concluded that there
was no evidence to suggest that stimulant medication increases the
likelihood of drug or alcohol use in adolescents. However, a
number of recent studies, drug abuse cases, and trends among
adolescents from various sources, indicate that methylphenidate use
may be a risk factor for substance abuse. Reports of adults
with ADHD have consistently demonstrated elevated rates of lifetime
psychoactive substance use disorders (PSUD).
In particular, 17 to 45% of ADHD adults had alcohol abuse problems
or dependence and 9 to 30% had drug abuse problems or dependence.
Recent prospective studies that have followed hyperactive children
and normal controls into adulthood have found that hyperactive
adults with a history of ADHD are more likely than controls to have
substance-use disorders.
Chronic preexposure to stimulants, including methylphenidate,
increases the rate of acquisition to cocaine self-administration in
rats.
Further, treatment with methylphenidate in childhood, predisposes
these same individuals as adults to cocaine’s reinforcing effects.
Clearly, this is an issue that needs further research.
Risks of Abuse
with Aging Treatment Population
In light of methylphenidate’s
abuse liability, it is important to note the tremendous increase in
availability of this substance and the expanded population
(adolescents and adults) receiving prescriptions for the treatment
of ADHD. Prescription data as well as aggregate production
quota information indicate that the use of methylphenidate has
increased substantially in the past few years. For example,
the aggregate production quota for methylphenidate has increased
from 1,361 kg in 1985 to 10, 410 kg n 1995 with the primary
increases occurring in the last five years (almost a 6-fold increase
since 1990). Epidemiological data indicate that approximately
85 to 90% of all prescriptions for methylphenidate are written for
young children and adolescents.
Abuse data indicate a growing
problem among school-age children. Children are remaining on
medication for longer periods of time, frequently into adolescence
and into adulthood. In addition, because so many families with
young children and adolescents are in daily contact with this
stimulant, a growing problem with abuse of methylphenidate in this
setting has been documented.
The aging treatment population is of major concern given evidence of
abuse by adolescents.
In addition, ADHD adults have a
high incidence of substance abuse disorders.
With three to five percent or more of today’s youth being
administered methylphenidate on a chronic basis, these issues are of
great concern.
References
Aarskog, D., Fevag, F., Klove, H., Stoa, K. and Thorsen, T. (1977).
J. Pediatr. 90: 136-139.
Ahmann, P.A., Waltonen, S.J., Olson, K.A., Theye, F.W., Van Erem,
A.J., and LaPlant, R.J. (1993). Placebo-Controlled Evaluation
of Ritalin Side Effects. Pediatrics. 9 (6):
1101-1106.
Akerman, P.T., Dykman, R.A. and Peters, J.E. (1977). Teenage
Status of Hyperactive and Non-hyperactive Learning Disabled Boys.
Am J Orthopsychiatry. 47: 577-596.
Aman,
M.G. and Werry, J.S. (1975). Methylphenidate in Children:
Effects Upon Cardiorespiratory Function on Exertion. Int J Ment
Health. 4: 119-131.
Arnett, E.N., Battle, W.E., Russo, J.V. and Roberts, W.C. (1976).
Intravenous Injection of Talc-Containing Drugs Intended for Oral
Use. American Journal of Medicine. 60: 711-718.
Ballard, J.E., Boileau, R.A., Sleator, E.K., Massey, B.H., and
Sprague, R.L. (1976). Cardiovascular Responses of Hyperactive
Children to Methylphenidate.
JAMA. 236 (25): 2870-2874.
Barkley, R.A. (1988). The Effects of Methlyphenidate on the
Interactions of Preschool ADHD Children With Their Mothers. J
Am Acad Child Adolesc Psychiatry. 27: 336-341.
Barkley, R.A., Karlsson, J., Strzelecki, E. and Murphy, J.V. (1984).
Effects of Age and Ritalin Dosage on the Mother-Child Interactions
of Hyperactive Children. J Consulting and Clin Psychol. 52:
739-749.
Barkley, R., McMurray, M., Edelbrock, C. and Robbins, K. (1990).
Side Effects of Methylphenidate in Children With Attention Deficit
Hyperactivity Disorder: A Systematic, Placebo-Controlled
Evaluation.
Pediatr. 86: 184-192.
Biederman, J., Faraone, S.V., Spencer, T., Wilens, T., Norman, D.,
Lapey, K.A., Mick, E., Lehman, B.K., and Doyle, A. (1993).
Patterns of Psychiatric Comorbidity, Cognition, and Psychosocial
Functioning Adults With Attention Deficit Hyperactivity Disorder.
Am J Psychiatry. 150 (12): 1792-1798.
Borg.,
E. (1961). Methylphenidate Addiction. Nord. Med. 65:211-213.
Bradley, C. (1937). The Behavior of Children Receiving
Benzedrine.
Am J Psychiatry. 94: 577-585.
Brooks, G.F., O’Donoghue, J.M., Rissing , J.P., Soapes,K., and
Smith, J.W. (1974). Eikenella Corrodens, A Recently Recognized
Pathogen: Infections in Medical-Surgical Patients and In
Association with Methylphenidate Abuse. Medicine. 53:
325-342.
Brown,
R.T., Wynne, M.E., and Slimmer, L.W. (1984). Attention-Deficit
Disorder and the Effect of Methylphenidate on Attention, Behavioral,
and Cardiovascular Functioning. J. Clin Psychiatry.
45(11): 473-476.
Brown,
R.T., Slimmer, L.W., and Wynne, M.E. (1984). How Much
Stimulant Medication is Appropriate for Hyperactive School Children?
JOSH. 54 (3): 128-130.
Brown,
W.A. (1977). Psychologic and Neuroendocrine Response to
Methylphenidate.
Arch Gen Psychiatry. 34: 1103-1108.
Brown,
W.A., Corriveau, D.P. and Ebert, M.H. (1978). Acute
Psychologic and Neuroendocrine Effects of Dextroamphetamine and
Methylphenidate.
Psychopharmacology. 58: 189-195.
Bryan,
V., Franks, L. and Torres, H. (1973). Pseudomonas Aeruginosa
Cervical Diskitis With Chondro-Osteomyelitis in an Intravenous Drug
Abuser.
Surg Neurol. 1: 142-144.
Carter, H.S. and Watson, W. A. (1994). IV
Pentazocine/Methylphenidate Abuse – The Clinical Toxicity of Another
Ts and Blues Combination.
Clin Tox. 32(5): 541-547.
Chait,
L.D. (1994). Reinforcing and Subjective Effects of Methylphenidate
in Humans. Behav Pharmacology. 5: 281-288.
Chillar, R.K., Jackson, A.L. and Alaan, L. (1982). Hemiplegia
After Intracarotid Injection of Methylphenidate. Arch. Neurol.
39: 598-599.
Conners, C.K. (1975). Controlled Trial of Methylphenidate in
Preschool Children With Minimal Brain Dysfunction. In:
Gittleman-Klein (Ed):
Recent Advances in Child Psychopharmacology. New York, Human
Sciences Press. Pp 65-78.
Cottler, L.B., Shillington, A.M., Compton III, W.M., Mager, D., and
Spitznagel, E.L. (1993). Subjective Reports of Withdrawal
Among Cocaine Users: Recommendations for DSM-IV. Drug
and Alcohol Dependence. 33: 97-104.
Dackis, C.A. and Gold, M.S. (1990). Addictiveness of Central
Stimulants.
Addictive Potential of Abused Drugs and Drug Classes. pp.
9-26.
Davidson, E.S., Lambert, N., Hartsough, C., and Schenk, S.
(submitted). Higher Incidence of Cocaine Use and Abuse in
Adult Subjects Exposed to Methylphenidate (Ritalin) as Children for
the Treatment for ADHD. (submitted).
Davy,
T., and Rodgers, C.L. (1989). Stimulant Medication and Short
Attention Span: A Clinical Approach. Developmental
and Behavioral Pediatrics. 10(6) 313-318.
Elenbaas, R.M., Waeckerle, J.F. and McNabney, W.K. (1976).
Abscess Formation as a Complication of Parenteral Methylphenidate
Abuse. JACEP. 5: 977-980.
Emmett-Oglesby, M.W., and Taylor, K.E. (1981). Role of dose interval
in the acquisition of tolerance to methylphenidate.
Neuropharmacology. 20: 995-1002.
Evans,
S.E. and Johanson, C.E. (1987). Amphetamine-like effects of
anorectic and related compounds in pigeons. J Pharmacol Exp Ther.
241: 817-825.
|